Location: Sydney NS, CA
Area: 4,640 m2
Project Cost: $20M CDN
Bio-containment level: BSL2
Share:
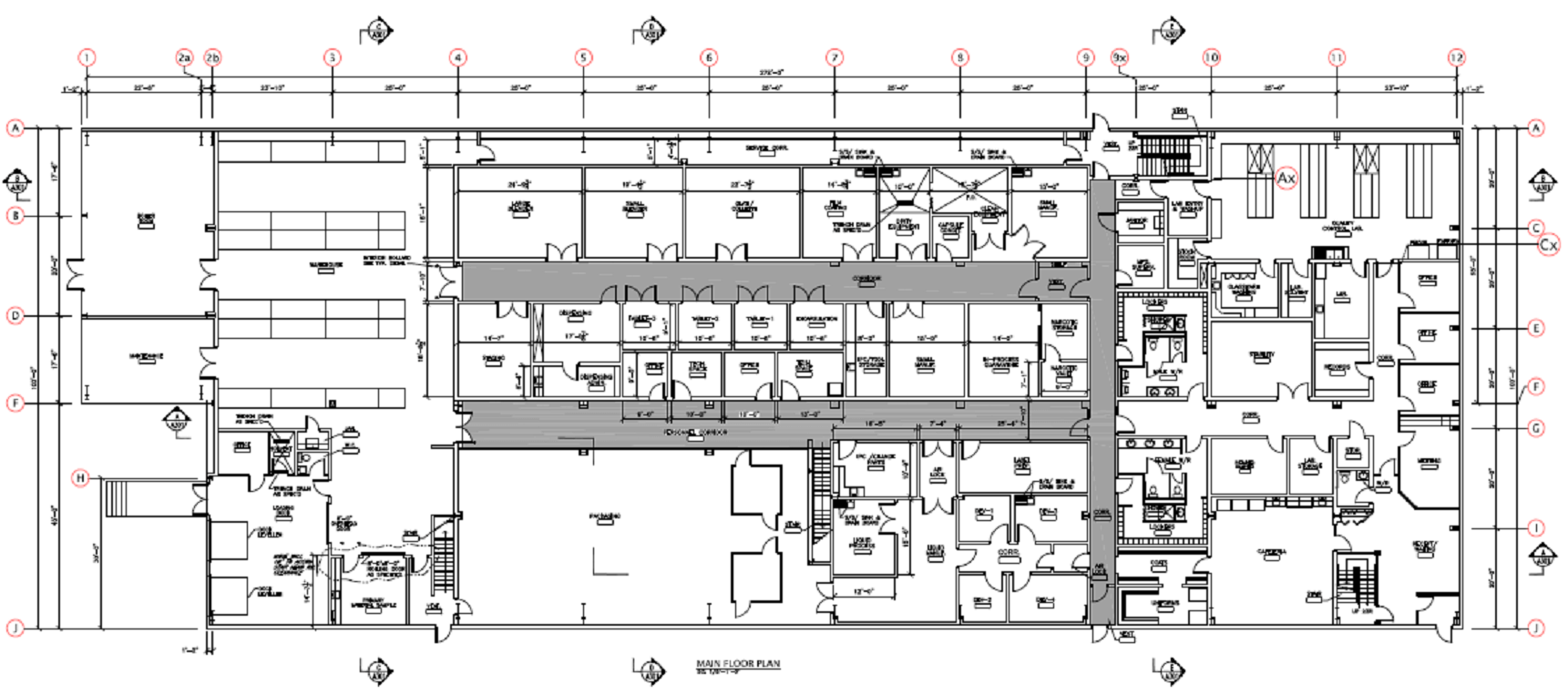
Keata Pharma is a state-of-the-art pharmaceutical manufacturing facility for solid and liquid dosage forms.
PharmEng provided design expertise by implementing a cost-effective GMP system to ensure that this facility will meet regulatory requirements internationally.
This facility encompasses GMP compliant QC laboratories, manufacturing and packaging suites for solid and liquid dosage forms.
The plant received its GMP establishment license in June.
Validation Activities Included:
- Validation Master Plan
- Utility Validation (HVAC, Water, Compressed Air, Vacuum)
- Equipment Validation
- Tablet Counter, Induction Sealer, Bottle Orientater
- Metal Detector, Inkjet Printer, Fluid Bed Dryer
- Coating Pans, Autoclave, Biosafety Cabinets,
- Fumehoods, Refrigerators, Thermal Mapping
- Process Validation
- Cleaning Validation
Responsibilities:
- Functional Flow Specifications
- Lab Planning
- Design
- Contract Documents
- Commissioning
- Compliance with FDA – cGMPs
- Validation
- Protocol Execution

Location:
Sydney NS, CA
Area:
4,640 m2
Project Cost:
$20M CDN
Bio-containment level:
BSL2
Share:






